
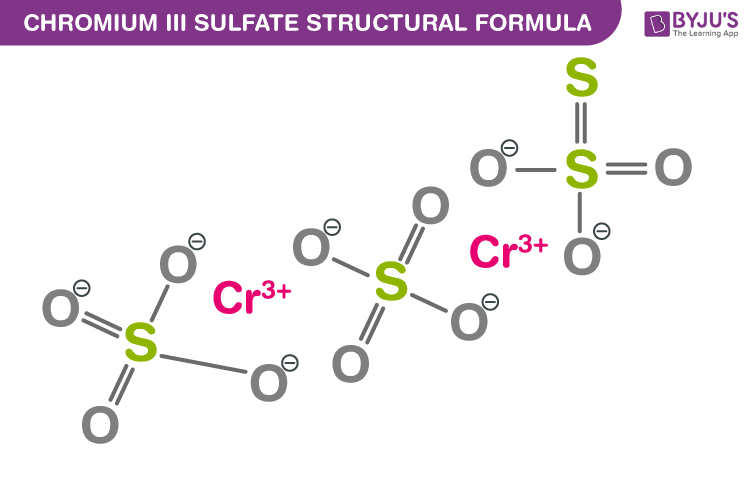
Question: This is the chemical formula for chromium (III) carbonate: C- (CO), Calculate the mass percent of oxygen in chromium (III) carbonate. 5 Interesting Facts About Green Chrome Oxide – Basstechintl. This problem has been solved Youll get a detailed solution from a subject matter expert that helps you learn core concepts. Write the chemical formula for each of the following substances: a) antimony (III) oxide b) nitrogen trifluoride c) lead (II) phosphate d) calcium hydroxide e) sodium phosphide f) cobalt (II) nitrate Write formulas for the following compounds.Stoichiometry of Ammonium Dichromate Decomposition –.It can also cause reproductive toxicity and is harmful to the unborn child.
#Chemical formula for chromium iii carbonate skin#
It may cause an allergic skin reaction and serious eye damage. Įxposure to chromium (iii) oxide through mouth and skin can cause acute toxicity. This is the chemical formula for chromium(III) carbonate: C-(CO), Calculate the mass percent of oxygen in chromium(III) carbonate. chromium(II) bromide, CrBr 2, and aqueous sodium carbonate, Na 2 CO 3. Cr2(CO3)3 Web15 ta Mej 2021 Chromium exists in two. Chromium(III) Bromide CrBr3 Molecular Weight - EndMemo Chromium(III) bromide is. It serves as an abrasive for polishing or stropping the edges of razors, knives, and surfaces of optical devices. Cations are always written first in the chemical formula of an ionic compound, followed by the anions.Because of its stability, chromium (iii) oxide is used as the green pigment in inks, glasses, and paints and as a colorant for ceramics, as well as producing a faint green tinge in glazes.Insoluble in acetone and alcohol, very slightly soluble in alkalies and acids

Molecular weight of Chromium(III) Phosphate - Convert Units What. When chromium (iii) oxide reacts with gaseous hydrogen sulfide, it yields chromium (iii) sulfide and water:Ĭr 2O 3 + 3H 2S → Cr 2S 3 + 3H 2O Properties and Characteristics of Chromium (III) Oxide General Propertiesĭry, light or dark green, fine crystalline powder What is the chemical formula for chromium(iii) phosphate (hint phosphate has a 3 charge). It reacts with dilute hydrochloric acid to form chromium (iii) chloride and water :Ĭr 2O 3 + 6HCl → 2CrCl 3 + 3H 2O Chromium (III) Oxide with Hydrogen Sulfide Molecular weight of Chromium - Convert Units WebThe isotopes of chromium range in atomic mass from 43 u ( 43 Cr) to 67 u. Chromium (iii) oxide, commonly called chromia and referred to as oxo(oxochromiooxy)chromium in IUPAC nomenclature, is an inorganic compound containing. Ammonium dichromate, on heating, decomposes into chromium (iii) oxide, water, and nitrogen :Ĭhromium (iii) carbonate is heated to form chromium (iii) oxide and carbon dioxide:Ĭr 2(CO 3) 3 → Cr 2O 3 + 3CO 2 Reaction with Other Compounds Chromium (III) Oxide with Hydrochloric Acid


 0 kommentar(er)
0 kommentar(er)
